Quantum numbers form the foundation for understanding atomic structure and electron behavior. These numbers describe…

Mastering Quantum Numbers: A Guide for Online Chemistry Tutors (Part 2)
In last week’s article, we looked at introducing quantum numbers using a condo analogy as well as relating those quantum numbers to the periodic table. This week, we’re going to discuss how to introduce the concept of orbitals and activities you can use to help your students solidify their understanding of quantum numbers.
3) Introduce Orbitals and Guidelines for Filling Orbitals
An orbital is a region of space where electrons can be found. Each orbital can hold a maximum of two electrons. The s-orbital has one suborbital, so it can hold a maximum of two electrons. The p-orbital has three suborbitals, so it can hold a maximum of six electrons. The d-orbital has five suborbitals, so it can hold a maximum of ten electrons, and the f-orbital has seven suborbitals so it can hold a maximum of 14 electrons.
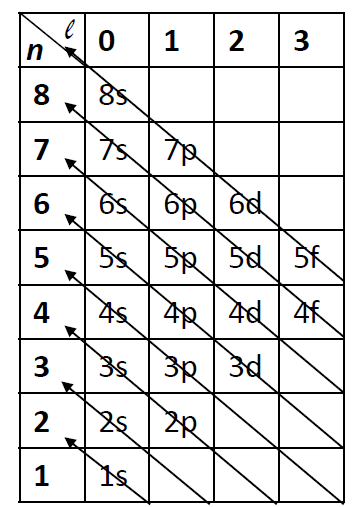
There are some rules for filling orbitals. The first is the Aufbau Principle. “Aufbau” is German for “building up,” and this states that every electron is added to the lowest energy level available until all electrons are accounted for. Here is a cheat sheet you can use to know which orbital is the next one to be filled.
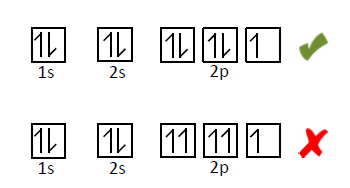
Next, the Pauli Exclusion Principle states that no two electrons in an atom can have the same four quantum numbers. This means that two electrons in the same orbital must have opposite spins!
Lastly, Hund’s Rule says that each suborbital of the same energy level must be occupied with one electron in the same direction before any pairing occurs.
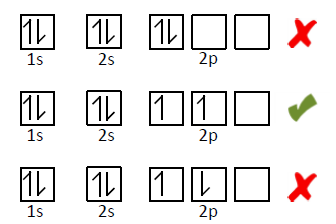
Using these three rules, you can teach your students how to fill in the orbitals with electrons!
4) Use Interactive Exercises to Help Students Solidify Understanding
Determine the Element from Quantum Numbers
Provide a set of quantum numbers and ask students to identify the corresponding electron. For example, you can ask students to identify the element that has quantum numbers 2, 1, -1, +½ for its last electron.
Students would analyze these numbers and see that the n = 2 which means that we’re working in the second energy level. l = 1, which is the p-block, so the possible elements would be between Boron and Neon. The third quantum number is ml = -1 and the spin is an up-spin (+½), so we know that ml = -1, 0, +1, and the up-spin arrows come first, so the element would be Boron.
To break this down a bit farther:
- ml = -1, ms = +½ (boron)
- ml = 0, ms = +½ (carbon)
- ml = +1, ms = +½ (nitrogen)
- ml = -1, ms = -½ (oxygen)
- ml = 0, ms = -½ (fluorine)
- ml = +1, ms = -½ (neon)
Identify the Quantum Numbers for an Element
Aside from giving the four quantum and asking students to figure out the element, you can also ask students to write the four quantum numbers for the last electron of any element. For example, you can give your students an element such as magnesium (Mg) and ask them to identify the four quantum numbers of the last electron.
Magnesium is in period three, so n = 3. It’s in the s-block, so l = 0. Since ml = -l to +l, then ml will only be 0. Therefore, Mg is the second element in the 3s orbital and it would have a negative spin, so ms = -½.
Therefore, the four quantum numbers for magnesium (Mg) would be (3, 0, 0, -½).
Valid Set of Quantum Numbers?
Provide a list of quantum numbers and ask your students to determine whether each set is valid. Remind them that a valid set must follow these rules:
- n (principal quantum number) is a positive integer: n = 1, 2, 3,…
- l (orbital angular quantum number) ranges from 0 to n-1
- ml (magnetic quantum number) ranges from -l to +l
- ms (spin quantum number) can only be +½ or -½
For example, (3, 2, -2, +½) is a valid set of quantum numbers, but (2, 2, 0, -½) is invalid because when n = 2, l cannot be equal to or greater than 2.
We hope you found this article along with the previous week’s article useful for introducing quantum numbers to your chemistry students. What other chemistry topics would you like to see in our weekly newsletter? Submit your requests in the comments below!