In last week’s article, we looked at introducing quantum numbers using a condo analogy as…

Mastering Quantum Numbers: A Guide for Online Chemistry Tutors (Part 1)
Quantum numbers form the foundation for understanding atomic structure and electron behavior. These numbers describe the unique coordinates of an electron in an atom, and no two electrons in one atom can have the same set of quantum numbers.
These four numbers specify the energy level, orbital shape, orientation, and spin of an electron. Many chemistry students find quantum numbers difficult to grasp because they were used to the Bohr-Rutherford model of the atom taught in prior chemistry courses. With the quantum numbers, students have to revamp how they envision atoms to be.
As an online chemistry tutor, you can help your students better understand quantum numbers by breaking down the concepts in simple terms, using analogies, and interactive exercises.
This two-part guide will help you simplify the concepts of quantum numbers so you can effectively help your students understand them.
1) Starting with the Basics by Introducing the Quantum Numbers with an Analogy
Before diving into the details, introduce the concept of quantum numbers by using the analogy of a condominium. Just as a condo has various floors, different suite styles, a certain number of rooms in each suite, and beds that you can sleep on either side of, quantum numbers have different characteristics that help you identify where an electron is within an atom.
Principal Quantum Numbers (n): Indicates the energy level and distance from the nucleus. The higher the quantum number, the farther the electron is away from the nucleus and the more energy and less stability it has.
- Condo analogy: The principal quantum number is like the floor of the condo.
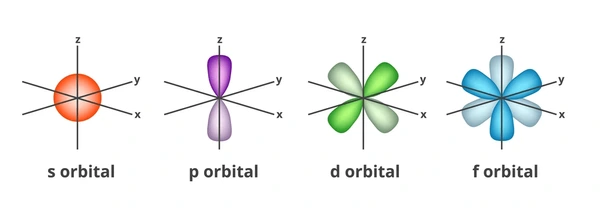
Orbital Angular Momentum Quantum Number (l): Describes the shape of the orbital (s, p, d, f). When l = 0, the orbital shape is s. When l = 1, the orbital shape is p. When l = 2, the orbital shape is d. When l = 3, the orbital shape is f. The shapes get more complicated as you go up the order.
- Condo analogy: s is like a simple room, p is a premium room, d is a deluxe room, and f is a fancy room.
Magnetic Quantum Number (ml): Specifies the orientation of the orbital. ml = -l to +l, so if l = 2, then ml = -2, -1, 0, +1, +2.
- Condo analogy: The magnetic quantum number is like a particular room within the condo unit.
Spin Quantum Number (ms): Denotes the direction of electron spin (+½ or -½). Two electrons in the same orbital must have opposite spins.
- Condo analogy: You can think of this as the side of the bed you sleep on.
2) Relate the Quantum Numbers to the Periodic Table
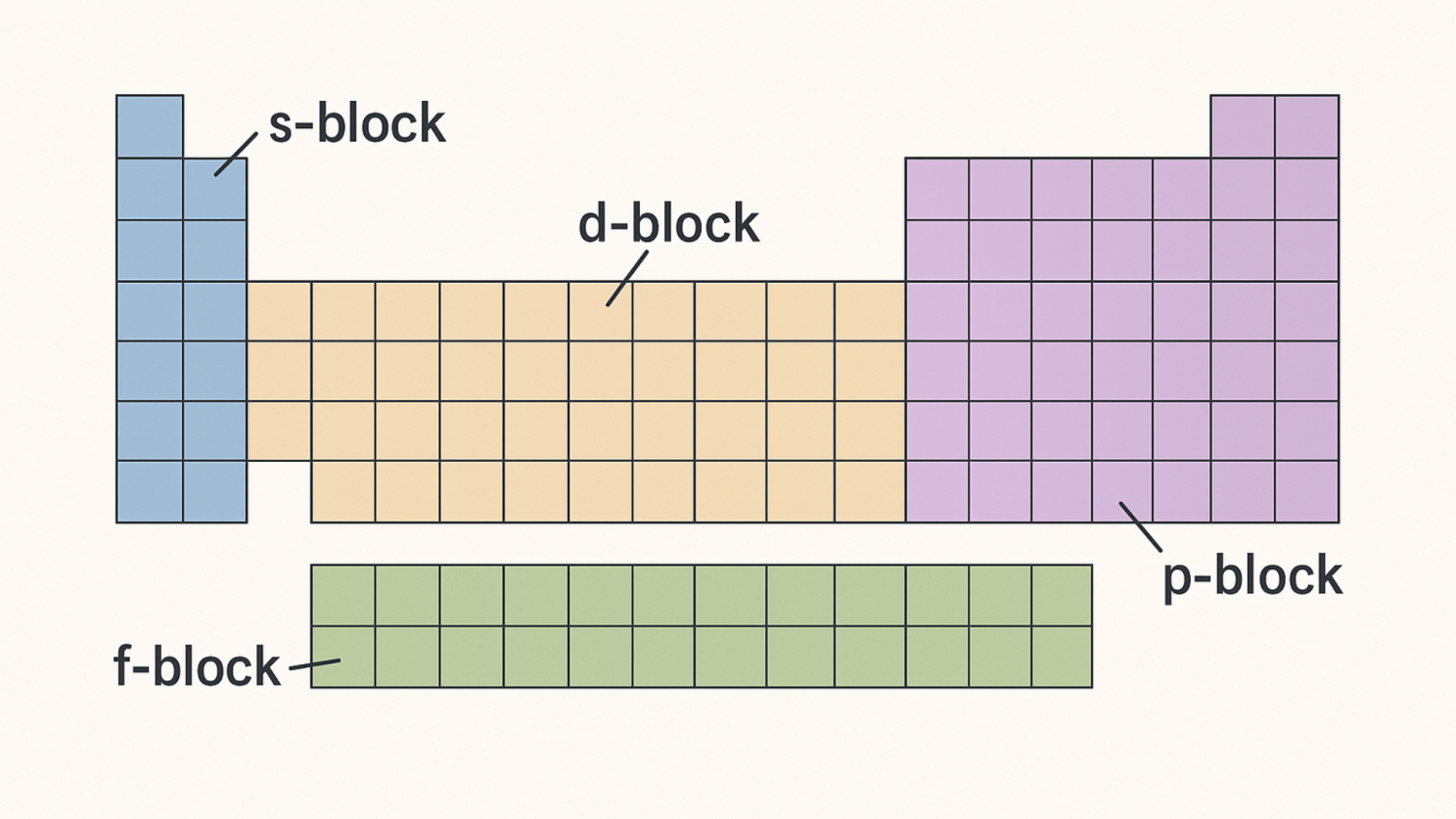
Explain to your students how the first two quantum numbers will let them know which section of the periodic table they’re working with. The s-block consists of groups 1 and 2, the p-block covers groups 13-18. The d-block represents the transition metals in groups 3-12, and the f-block points to the lanthanides and actinides at the bottom.
The principal quantum number (n) relates to the period number. For the s– and p-blocks, the n value starts from 1. For the d-block, the n value begins at 3, and for the f-block, the n value begins at 4.
This introduction will give your students some ideas of what the four quantum numbers represent. Stay tuned for next week’s Part 2 article that will explain the concepts of orbitals and energy diagrams as well as provide exercises you can use with your students to solidify their understanding of this chemistry topic.