In last week’s article, we looked at introducing quantum numbers using a condo analogy as…

A Guide for Teaching Rates of Reaction in Chemistry
Much of what we come across day to day involve chemical reactions, from the food we cook to the rusting of metals and even the processes happening inside our bodies. Understanding how and why these reactions occur at different speeds is a fundamental part of chemistry, which is why it’s important to cover rates of reaction in chemistry courses.
By understanding the factors that influence reaction rates and the principles behind them, students gain insight into controlling and predicting chemical behavior. Here are eight key topics to cover when teaching about rates of reaction.
1) Definition of Reaction Rate
The rate of a chemical reaction refers to how quickly reactants are converted into products. It is measured by the change in concentration of reactants or products over time. Mathematically it can be expressed as: Rate of reaction = Change in concentration / Change in time.
2) Factors Affecting the Rate of Reaction
Several factors influence how fast or slow a reaction proceeds. These include:
- Concentration of Reactants: Higher concentration means more particles are available to collide, increasing the reaction rate.
- Temperature: Raising the temperature gives particles more energy, leading to more frequent collisions at higher energy.
- Surface Area: Increasing the surface area (E.g. using powdered reactants vs. solid pieces) exposes more particles to collision, speeding up the reaction.
- Catalysts: Catalysts speed up reactions by providing an alternative pathway with lower activation energy, without being consumed by the reaction.
- Pressure (for gases): Higher pressure forces gas molecules closer together, resulting in more collisions and a faster reaction rate.

3) Activation Energy (Ea)
The activation energy is the minimum energy required for reactants to collide successfully and form products. It represents the energy barrier that must be overcome for a reaction to proceed. The lower the activation energy, the faster the reaction.
4) Collision Theory
Collision Theory explains that reactions occur when particles collide with sufficient energy and correct orientation. Three main principles of this theory are:
- Frequency of Collisions: The more frequent the collisions between reactant particles, the higher the rate of reaction.
- Activation Energy: Not all collisions lead to a reaction. Only those collisions that involve particles with enough energy to overcome the activation energy barrier result in product formation.
- Correct orientation: Particles must collide with the proper alignment for a reaction to occur. Think of this like a lock and key. The key can only enter the lock in one orientation.
5) Rate Laws and Rate Equations
A rate law expresses the relationship between the rate of reaction and the concentration of reactants. It has this expression: Rate = k[A]m[B]n
- k is the rate constant
- [A] and [B] are the concentrations of the reactants
- m and n are the orders of reaction with respect to each reactant, indicating how the concentration of each reactant affects the reaction rate. (The values of m and n must be determined experimentally.)
6) Order of Reaction
The order of reaction describes how the rate depends on the concentration of the reactants:
- Zero-order reaction: The rate is constant and independent of the concentration of reactants.
- First-order reaction: The rate is directionally proportional to the concentration of one reactant.
- Second-order reaction: The rate is proportional to the square of the concentration of one reactant or to the product of two reactant concentrations.

7) Graphs and Reaction Rates
There are two common types of graphs that show reaction rates:
- Concentration vs. Time graph: This graph shows how the concentration of reactants or products changes over time. A steeper slope indicates a faster reaction.
- Rate vs. Concentration graph: This graph helps to determine the order of reaction by plotting the rate against the concentration of one of the reactants. The shape of the graph reveals whether the reaction is zero, first, or second order with respect to that reactant.
8) Temperature and the Arrhenius Equation
The relationship between temperature and reaction rate is shown by the Arrhenius equation:
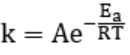
- k is the rate constant
- A is the pre-exponential factor (related to the nature of the reaction)
- Ea is the activation energy
- R is the gas constant (8.314 J/mol K)
- T is the temperature in Kelvin
As temperature increases, the exponential factor grows, meaning the rate constant, and hence, the reaction rate increases. It also shows that if the activation energy is decreased, then the rate constant, as well as the reaction rate, increases.
If you are a chemistry teacher or tutor, make sure you cover these topics when teaching about rates of reaction!